Scientists consider this to be hope in the race to develop a vaccine, but also emphasize that it cannot be confirmed whether vaccines from Pfizer and other candidates can prevent the pandemic.
`We still have to look at the final data, but this doesn’t dampen my enthusiasm. The information is great. I hope I’m not in the placebo group,` Florian Krammer, virus expert at
The vaccine contains information mRNA, the task of instructing human cells to produce nCoV spike protein, stimulating the immune system to produce antibodies against the virus.
According to Eric Topol, a cardiologist and director of the Scripps Research Translational Institute, the vaccine may no longer be 90% effective when the trial ends, but its protection level will still be above 50%, exceeding the safety threshold.
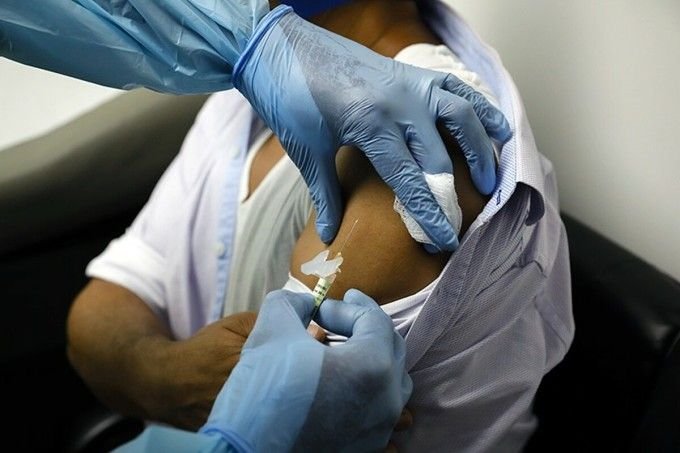
Volunteers testing the Covid-19 vaccine from pharmaceutical company Pfizer and BioNTech in Brazil.
Experts say the current question is: Can BNT162b2 only fight mild cases of nCoV infection, or will it prevent the disease from becoming more serious?
It is also unclear whether the injections can block the transmission of the virus in Covid-19 patients with very mild symptoms.
The other missing piece in the vaccine puzzle is its effect in different target groups.
But Dr. Paul Offit added that if the trial attracts enough participants from minority groups, scientists can generalize the vaccine’s effects from the overall effectiveness.
It is unclear how long immunity formed by the vaccine can last.
To explain this, scientists need to extend the experiment for a few more months, analyzing the reactions of early-stage volunteers.
On November 9, pharmaceutical company Pfizer and partner BioNTech announced that their vaccine was 90% effective in preventing Covid-19.